NIKKISO CO., LTD.
Introduction of SAP MES "SAP® Manufacturing Execution (SAP ME) / SAP® Manufacturing Integration and Intelligence (SAP MII)" at a Hemodialysis machines production site
Provision of technical support by Fujitsu to improve conformance with various countries’ regulations and to enhance quality and operational efficiency

NIKKISO, a pioneer in the field of dialysis machines, manufactures hemodialysis machines at its Kanazawa Plant Medical Factory. In recent years, it has become an urgent issue to respond to ever-tightening domestic and overseas regulations. The company departed from paper-based manufacturing by introducing "SAP® Manufacturing Execution (SAP ME) / SAP® Manufacturing Integration and Intelligence (SAP MII)," a manufacturing execution system, in order to store an enormous number of documents, strengthen traceability, and tackle challenges, such as enhancing quality and operational efficiency as well as visualizing the production site.
This introduction aims to achieve coordination with the already introduced SAP ERP, and to support variant configuration for build-to-order manufacturing in addition to planned manufacturing. While forming consensus with the production site, the company also created new workflows with technical support from Fujitsu. The new system is expected to help to standardize tasks, to visualize the production site, and to dramatically reduce the required man-hours.
- ChallengesNeed to improve conformity with domestic and overseas regulations
- BenefitsThe company introduced SAP® Manufacturing Execution (SAP ME) / SAP® Manufacturing Integration and Intelligence (SAP MII) and transitioned away from paper-based manufacturing. They intend to promote paperless manufacturing and to strengthen traceability.
- ChallengesNeed to support variant configuration for planned and build-to-order manufacturing
- BenefitsFujitsu's add-on development made it possible to support variant configuration without changing the existing bills of materials (BOMs) and work procedures.
- ChallengesNeed to enhance quality and operational efficiency, and to visualize the condition of the shop floor
- BenefitsThe company transitioned from paper to digital, improved workflows, enhanced quality by standardizing tasks, dramatically reduced man-hours by eliminating posting work, and expedited work evaluations by visualizing the production site.
Background
Urgent needs to improve conformity with strict domestic and overseas regulations,
to enhance quality and operational efficiency, and to visualize the production site
Since its establishment in 1953, NIKKISO has created markets as a pioneer in fields that require high expertise. It has explored harsh conditions under which no failure is tolerated and technically challenging areas, such as specialized industrial pumps, aviation parts made of carbon fiber reinforced plastic (CFRP), hemodialysis machines, and products that use Deep UV-LEDs, and the company has contributed to solving social issues by employing creative ideas and advanced technologies.
In 1969, NIKKISO made it possible to manufacture hemodialysis machines in Japan. The achievements made by its medical equipment business in tandem with the development of dialysis constitute the history of dialysis in Japan. The company holds a greater than 50% share in the hemodialysis machine market in Japan and operates its business worldwide. What is required to drive further growth in the medical equipment business is to improve conformity with domestic and overseas regulations, to enhance quality and operational efficiency, and to visualize the production site. Mr. Izumi Takayoshi, General Manager of the company's Kanazawa Plant Medical Factory, noted that departing from a paper culture was crucial for solving these important challenges.
"As this factory manufactures hemodialysis machines, it is necessary to store an enormous number of documents, such as manufacturing records and processes, and to strengthen traceability. It was difficult to meet the requirements imposed by strict regulations with conventional paper-based manufacturing operations. Utilization of IT is crucial for responding to global environmental changes in the dialysis business in a speedy, flexible manner. It was urgent for us to realize data-based manufacturing in order to enhance quality and operational efficiency as well as to visualize the production site by utilizing IT."
In 2016, NIKKISO began to consider the introduction of a manufacturing execution system (MES) that visualizes production site information and computerizes procedure manuals and records through digitization in order to transform the manufacturing that supports its dialysis business.
 Mr. Izumi Takayoshi
Mr. Izumi Takayoshi
Executive Officer
General Manager
Kanazawa Plant Medical Factory
Medical Division
NIKKISO CO., LTD.
Details
The challenges of storing a multitude of records and strengthening traceability
Aiming to visualize the production site and thoroughly observe procedure manuals
Mr. Izumi reviewed the issues with the existing paper-based production system regarding conformity with various regulations. "As of 2016, the factory was already short of space due to storing paper information. Improved conformity with domestic and overseas regulations resulted in a further increase in the amount of paper documentation, making our transition to paper-less manufacturing inevitable in order to free up some space and to make it easier to find records. In addition, digitization-based traceability improvements were necessary to ensure that the histories of parts used in products were recorded and stored."
Mr. Sugimoto Kazuhiko of the Production Control Department, who is supervisor of the project, declared that visualization of the production site needed to be realized. "At the production site, process progress varies depending on various factors on a daily basis. Strengthening improvement activities to visualize the production site for causal analysis and issue resolution was required to further enhance quality and operational efficiency."
In addition, Mr. Sugimoto said that avoiding the risks that arise from paper-based work was also a challenge. "Not only skilled workers, but also new recruits sometimes carried out tasks without reading the procedure manuals because they had grown too accustomed to the production site. Thorough observance of procedure manuals and standardization of tasks were our immediate issues."
 Mr. Sugimoto Kazuhiko
Mr. Sugimoto Kazuhiko
Project supervisor
General Manager
Production Control Department Kanazawa Plant
Medical Division
NIKKISO CO., LTD.
Key points for adoption
Focus on five points for selecting an MES product
including the minimization of development man-hours and a flexible production system
There were five key points for NIKKISO when selecting an MES product to solve its problems with paper-based manufacturing.
First was to minimize the development man-hours required to integrate with the backend system with the MES. "As we had already introduced SAP ERP, we selected SAP® Manufacturing Execution (SAP ME) / SAP® Manufacturing Integration and Intelligence (SAP MII), which is an SAP MES, as the first candidate from the perspective of reducing the development man-hours. In addition, manufacturing in coordination with manufacturing equipment and automated collection of inspection data were also advantages." (Mr. Sugimoto)
Second was to support variant configuration for flexible build-to-order and planned manufacturing in order to meet the different specifications required by medical institution customers. "Though there are standard models for hemodialysis machines, specifications vary depending on the customer's objective and system environment. The key point was whether the variant configuration that we had used thus far could be used in the system. We appreciate that the close coordination of SAP ERP and SAP ME, including masters, makes it possible to automatically select appropriate work procedure manuals and checklists for different specifications, and variant configuration can be achieved by following procedure manuals." (Mr. Sugimoto)
Third was to visualize the production site. "An advantage of SAP MII is that it can retrieve data from SAP ME and SAP ERP and visualize it in real time," said Mr. Miyawaki Kazuyoshi of the Global Information Management Department, who is in charge of the project management office (PMO).
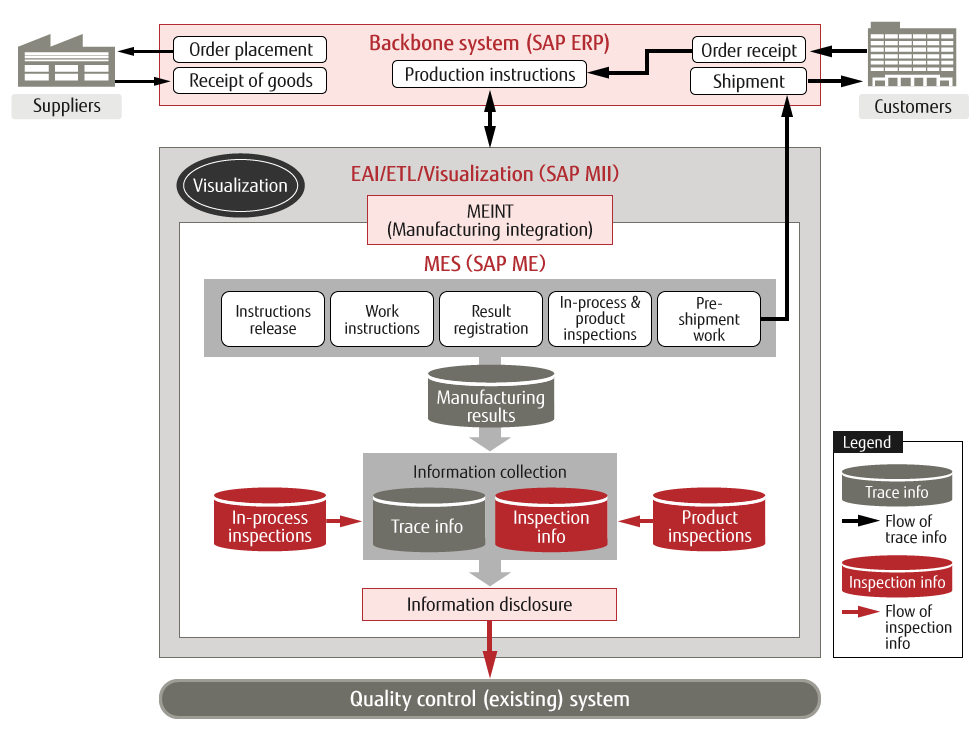 System Diagram
System Diagram
Fourth was to prevent tampering with the electronic records. "SAP ME logs requests, including who accessed what at which dates and times, and permits only authorized people to make changes, thereby making it possible to prevent tampering," noted Mr. Haraguchi Akihiro, Project Manager, of the Molding Department.
Fifth was the efficient system introduction. "The key point was whether the standard functions of SAP ME were suitable for our tasks." (Mr. Haraguchi)
 Mr. Miyawaki Kazuyoshi
Mr. Miyawaki Kazuyoshi
Project Management Office
General Manager
Global Information Management Department
NIKKISO CO., LTD.
 Mr. Haraguchi Akihiro
Mr. Haraguchi Akihiro
Project Manager
General Manager
Molding Department
Kanazawa Plant
Medical Division
NIKKISO CO., LTD.
Introduction process
New workflows created with technical support from Fujitsu
to align tasks with standard functions to improve operational efficiency
NIKKISO conducted a proof of concept (PoC) of SAP® Manufacturing Execution (SAP ME) / SAP® Manufacturing Integration and Intelligence (SAP MII) over three months starting in January 2018. "We confirmed basic usage with support from Fujitsu and SAP, and we asked the production staff to try out the system." After gaining confidence in complete introduction through the PoC, they decided to adopt SAP® Manufacturing Execution (SAP ME) / SAP® Manufacturing Integration and Intelligence (SAP MII) after comprehensive considerations, including the aforementioned five points. They selected Fujitsu as their implementation partner because of its wealth of experience with SAP products as well as technical and comprehensive capabilities.
In April 2018, NIKKISO began to define requirements. Regarding its policy on this introduction, Mr. Nakao Michiharu of the Manufacturing Engineering Department, the project leader, said, "We developed the policy of improving workflows with the aim of enhancing operational efficiency in alignment with the standard functions of packages, thereby introducing the system efficiently. This is because doing so reduces upfront costs and also, the more add-ons we develop, the more difficult it would be to modify them for updates."
The key to successfully introducing MES is to form consensus with the production site that is responsible for manufacturing. Mr. Nakao said that he requested that the production site’s representative join the project team. "As business-process reform was required to occur at the same time as the introduction of SAP ME, we had discussions with the person in charge of the production site. We listed the workflows used before the introduction on a large sheet of paper, considered whether it would be possible to change those workflows in order to improve operational efficiency in alignment with the standard functions, and created new workflows to be used after the introduction. We also requested Fujitsu to participate in the discussions, and they provided us with specific, thorough advice and proposals regarding each function from the viewpoint of the production site, such as that certain functions change workflows in certain ways and can also be used for other purposes."
What troubled NIKKISO in terms of system construction was the support of variant configuration that they were emphasizing. "As SAP ME did not support the bills of materials (BOMs) that we use, we had to make major changes to the BOMs on the ERP and work procedures in order to support variant configuration with standard functions. We requested that Fujitsu develop add-ons in order to avoid prolonging the project, and they successfully realized variant configuration without us needing to change our operations."
It will become mandatory to observe the Computerized System Validation (CSV)* guidelines required by regulations in various countries. Fujitsu compared the CSV guidelines against Fujitsu's solution-based system development engineering methodology (SDEM) and introduced the system to ensure strict observance of the CSV guidelines while recording evidence.
* CSV: The process of validating that a computerized system that integrates a computer system and business processes functions as intended by the user, and documenting evidence of said validity.
Benefits of the solution and future outlook
Standardized tasks as well as enhanced quality and productivity
Dramatic reduction in man-hours expected
In December 2019, the SAP® Manufacturing Execution (SAP ME) / SAP® Manufacturing Integration and Intelligence (SAP MII)-based manufacturing execution system went live for some products at the hemodialysis machine production site, where high quality is required. Mr. Nakao said that the workflows changed dramatically upon the transition from paper to digital. "At the production site, a task is carried out by following a procedure manual displayed on a tablet. Workers have to tap ‘Task completed' for each step; otherwise, they cannot proceed to the next task. In addition, we were able to standardize tasks by carrying out procedures appropriate for the various steps, thereby enhancing quality and productivity throughout the entire factory."
Mr. Nakao said that they were seeing progress with visualization of the production site, which has been a pending issue. "As supervisors and group leaders as well as workers at the production site can see the progress of tasks using tablets and submonitors, work decisions can be made swiftly depending on the site’s situation. In addition, we made it possible to visualize the production site for factory visitors by viewing a large monitor, which also serves to foster trust."
As digitization has made it possible to search for various items and to utilize needed information immediately, NIKKISO can now promptly confirm quality conditions and respond to customers inquiries. The company also expects a dramatic reduction in the number of man-hours thanks to the elimination of the need to prepare work procedure manuals and checklists as well as to post inspection data. In addition, the paperless system has helped to reclaim some space inside the factory.
Regarding future prospects, Mr. Izumi commented, "The complete application of SAP® Manufacturing Execution (SAP ME) / SAP® Manufacturing Integration and Intelligence (SAP MII) is yet to begin. We will also pursue ease of use by reflecting the production site’s opinions. In addition to improving the accuracy of procedure manuals, we are also considering means to convey procedures in an easier-to-understand manner by using 3D graphics and video. Further enhancing quality and strengthening improvement activities by utilizing accumulated data is another important topic. I expect Fujitsu to provide prompt support in close coordination with SAP, proactively make proposals that utilize SAP® Manufacturing Execution (SAP ME) / SAP® Manufacturing Integration and Intelligence (SAP MII) functions, and keep us up-to-date with the latest information, such as other companies' cases."
NIKKISO uses innovative technologies to create new value and solve social issues. Going forward, Fujitsu will continue to support NIKKISO's initiatives by utilizing its advanced technologies and comprehensive strengths.
 Mr. Nakao Michiharu
Mr. Nakao Michiharu
Project leader
Chief
Manufacturing Engineering Department, Kanazawa Plant
Medical Division
NIKKISO CO., LTD.
NIKKISO CO., LTD.
| Head Office | Yebisu Garden Place Tower 22nd Floor, 20-3, Ebisu 4-Chome, Shibuya-ku, Tokyo 150-6022, Japan |
|---|---|
| President & CEO | Toshihiko Kai |
| Date of Establishment | December 26, 1953 * The registered date of establishment is March 7, 1950. |
| Paid-in Capital | JPY 6,544,339,191 |
| Number of Employees | 2,140 (Consolidated: 8,708) |
| Website | https://www.nikkiso.com/ |
[Published March 2021]
