Fujitsu Embarks on Joint Research with National Cancer Center Japan to Create New Services for Drug Development and Clinical Trials
Research draws from real-world data sources including electronic medical records
Fujitsu Limited
Tokyo, March 18, 2021
Fujitsu today announced it has concluded a comprehensive collaborative research agreement with National Cancer Center Japan. The joint research will center on exploring ways that real-world data (1), including medical information from electronic records, can be leveraged to contribute to the development of new drugs, clinical trials, and preventive medicine in pharmaceutical companies.
Through its joint research with the National Cancer Center, Fujitsu aims to create new value through a wide range of information and analysis services by creating an anonymized personal health record scheme (2) (PHR), which includes case study information and health information on patients visiting the National Cancer Center Hospital East. Fujitsu additionally seeks to create a platform for providing pharmaceutical companies with data that can be used for drug development and preventive medicine. Fujitsu will establish a new model to process medical information from electronic medical records into highly secure statistical data to improve the efficiency of clinical trials and supports active participation in international joint clinical trials by promoting the adaptation of clinical trial data in Japan to global standards. (3).
With an eye to contributing to the wellbeing of people in society, Fujitsu will work to improve the development speed and quality of pharmaceuticals and other products that promote the health of consumers. In turn, Fujitsu will play a role in resolving issues such as the time required for clinical trials and costs, create a variety of services that contribute to areas such as preventive medicine and individualized cancer medicine, and implement these services by the end of fiscal 2021.
Background
In recent years, real-world data has been attracting increased attention in the healthcare field, and it is beginning to be used in a wide range of fields globally—from drug approval applications to the development of medical devices and preventive medicine. A variety of different data types are being used to these ends, ranging from patient electronic medical record information and medical examination information, to daily measurement data such as weight and body composition monitors (4), as well as vital information such as temperature and blood pressure that can be obtained from smart devices.
Despite the potential for such data, however, challenges remain around extracting useful information from unstructured sources like doctors’ notes, etc., and it has become necessary to develop new platforms and data extraction technologies to effectively and securely streamline the use of valuable medical data. To this end, Fujitsu has concluded a comprehensive agreement with the National Cancer Center with the ultimate goal of contributing to the speedy development of pharmaceuticals and clinical trials by pharmaceutical companies that promote the health of consumers and will start joint research in this field.
About the Joint Research
1. Overview
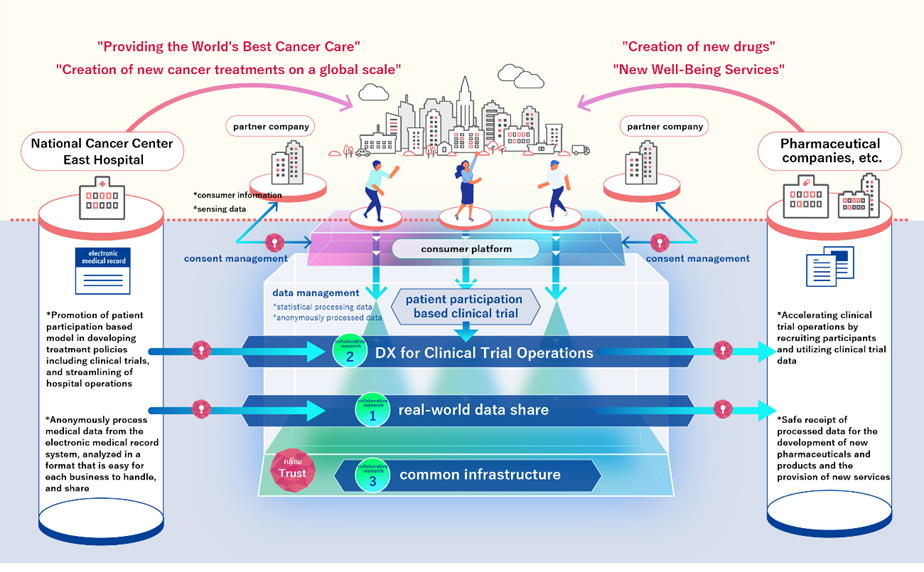
In collaboration with the National Cancer Center, Fujitsu is implementing three projects to utilize real-world data at the National Cancer Center Hospital East.
- Verification of utilization of real-world data (real-world data share)
- In addition to medical information from the electronic medical record systems, which has been difficult to use until now, information on case studies and information on the health of local patients (PHR) is processed into a format that is easy for pharmaceutical companies to handle and provided as secure, high-quality data. The effectiveness of using real-world data in developing new AI technologies to support diagnoses and treatment will also be verified.
- Establishment and validation of new clinical trial service models (DX for Clinical Trial Operations)
- Natural language analysis technology will be applied to the analysis of medical information in the electronic medical record system in order to establish a new clinical trial service model that can automatically identify appropriate clinical trial patients with overwhelming speed and accuracy. This process is presently performed manually with reference to medical information in the electronic medical record system (clinical trial patient recruitment). Through this study, the effectiveness of the system in significantly reducing the cost and duration of clinical trials, including improving the efficiency of in-hospital operations will be verified.
- Fujitsu will promote efforts to adapt clinical trial data in Japan to global standards and support active participation in international clinical trials.
- Establishment of a platform for safe and secure use of medical data (common platform)
- In order to carry out these two verifications, Fujitsu will develop a platform that can safely and securely utilize medical data by converting medical information recorded as text data in the electronic medical record system into a format that can be utilized as data. This platform utilizes technology developed by Fujitsu Laboratories, Ltd., as well as data extraction technology that is being newly developed.
 Overview of the Joint Research Project
Overview of the Joint Research Project2. Role of Fujitsu, National Cancer Center
Fujitsu
- Development of technologies and platforms needed to utilize real-world data
- Verifying streamlining of clinical trial operations with patient recruiting function
- Coordination with national policies for the utilization of PHRs (5)
National Cancer Center
- Provision of medical data at National Cancer Center Hospital East
- Provision of know-how on clinical practice, clinical research, and clinical trials
- Verification of the contents of joint research
3. Period of Joint Research
March 16, 2021 ~ March 31, 2023
4. Location
National Cancer Center Hospital East and Exploratory Oncology Research & Clinical Trial Center
Future Plans
In the future, Fujitsu will not only collaborate with pharmaceutical companies, but also consider working with various companies that provide wellbeing-related products and services to consumers in Japan and throughout the world. Based on the results of this joint research, by the end of fiscal 2021 Fujitsu will develop platforms and services that enable the safe and secure use of real-world data and provide wellbeing to consumers. Through the provision of these platforms and services to pharmaceutical companies, Fujitsu aims to contribute to the streamlining of drug development and clinical trials.
- [1]real-world data:
Data generated by daily medical care and personal health management. - [2]PHR:
A system in which individuals collect and manage their own medical information and other health-related information. Refers to information that can be subject to disclosure control at the discretion of the individual. - [3]international joint clinical trials:
A clinical trial conducted simultaneously in multiple countries or regions. - [4]weight and body composition monitors:
a measuring instrument that measures weight and body fat percentage and visceral fat levels etc. - [5]National policies:
For example, policies led by Data Health Reform Promotion Headquarters of the Ministry of Health, Labour and Welfare such as "Health, Medical, and Nursing Care Information Utilization Study Group".
About Fujitsu
Fujitsu is the leading Japanese information and communication technology (ICT) company offering a full range of technology products, solutions and services. Approximately 130,000 Fujitsu people support customers in more than 100 countries. We use our experience and the power of ICT to shape the future of society with our customers. Fujitsu Limited (TSE:6702) reported consolidated revenues of 3.9 trillion yen (US$35 billion) for the fiscal year ended March 31, 2020. For more information, please see www.fujitsu.com.
Press Contacts
Public and Investor Relations Division
Inquiries
Company: Fujitsu Limited
All company or product names mentioned herein are trademarks or registered trademarks of their respective owners. Information provided in this press release is accurate at time of publication and is subject to change without advance notice.
Date: 18 March, 2021
City: Tokyo, Japan
Company: Fujitsu Limited